Add an exciting new glaucoma technology to your cataract surgery.
 The cataract surgery you are scheduled for is a good example of how innovations can make a difference utilizing recently developed technology that will help us improve your vision. Now we are able to add another step to your cataract surgery that allows us to treat your open-angle glaucoma in a completely new way. Most patients like you will spend the rest of your lives putting one, two or even three different kinds of drops in every day. All of these drops are not only be inconvenient, but potentially very expensive. The iStent Trabecular Micro-Bypass Stent is designed to reduce your eye pressure and you can have it done at the same time you have cataract surgery.
The cataract surgery you are scheduled for is a good example of how innovations can make a difference utilizing recently developed technology that will help us improve your vision. Now we are able to add another step to your cataract surgery that allows us to treat your open-angle glaucoma in a completely new way. Most patients like you will spend the rest of your lives putting one, two or even three different kinds of drops in every day. All of these drops are not only be inconvenient, but potentially very expensive. The iStent Trabecular Micro-Bypass Stent is designed to reduce your eye pressure and you can have it done at the same time you have cataract surgery.
iStent: the world’s smallest medical implant delivers big results in mild-to-moderate open-angle glaucoma.
Open-angle glaucoma is very common, although many people are unaware of their condition, especially in the early stages, when their vision may be unaffected. Open-angle glaucoma is characterized by an increase in the intraocular pressure (IOP) of your eye. This pressure is caused by the obstruction of fluid flow within the eye. Too much fluid raises pressure, which can cause the gradual loss of vision. And while glaucoma moves slowly, its damage is irreparable.
The world’s tiniest medical device—iStent—is less than 1 millimeter in size. But the size of iStent is only part of its story. By increasing the eye’s ability to drain fluid, this technology is designed to reduce the pressure in your eye.
In a U.S. clinical study, 68% of glaucoma patients who received iStent remained medication free at 12 months.
How iStent works is elegantly simple:
- If you have glaucoma, over time the eye’s natural drainage system becomes clogged
- iStent creates a permanent opening through the blockage to improve the eye’s natural outflow
- Restoring this mechanism lowers and controls pressure within the eye
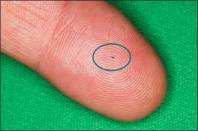 iStent: managing glaucoma while treating your cataracts
iStent: managing glaucoma while treating your cataracts
iStent is implanted during your cataract surgery procedure. Once implanted, iStent will begin working to safely and effectively manage pressure. What’s more, patients who receive iStent may experience a reduction in glaucoma medications; but this will be at the discretion of your physician.
The iStent® Trabecular Micro-Bypass Stent (Models GTS100R and GTS100L) is indicated for use in conjunction with cataract surgery for the reduction of intraocular pressure (IOP) in adult patients with mild-to-moderate open-angle glaucoma currently treated with ocular hypotensive medication.
The iStent® is contraindicated in eyes with primary or secondary angle closure glaucoma, including neovascular glaucoma, as well as in patients with retrobulbar tumor, thyroid eye disease, Sturge-Weber Syndrome or any other type of condition that may cause elevated episcleral venous pressure.
Gonioscopy should be performed prior to surgery to exclude PAS, rubeosis, and other angle abnormalities or conditions that would prohibit adequate visualization of the angle that could lead to improper placement of the stent and pose a hazard. The iStent® is MR-Conditional meaning that the device is safe for use in a specified MR environment under specified conditions, please see label for details.
The surgeon should monitor the patient postoperatively for proper maintenance of intraocular pressure. The safety and effectiveness of the iStent® has not been established as an alternative to the primary treatment of glaucoma with medications, in children, in eyes with significant prior trauma, chronic inflammation, or an abnormal anterior segment, in pseudophakic patients with glaucoma, in patients with pseudoexfoliative glaucoma, pigmentary, and uveitic glaucoma, in patients with unmedicated IOP less than 22 mmHg or greater than 36 mmHg after “washout” of medications, or in patients with prior glaucoma surgery of any type including argon laser trabeculoplasty, for implantation of more than a single stent, after complications during cataract surgery, and when implantation has been without concomitant cataract surgery with IOL implantation for visually significant cataract.
The most common post-operative adverse events reported in the randomized pivotal trial included early post-operative corneal edema (8%), BCVA loss of ≥ 1 line at or after the 3 month visit (7%), posterior capsular opacification (6%), stent obstruction (4%) early post-operative anterior chamber cells (3%), and early post-operative corneal abrasion (3%). Please refer to Directions for Use for additional adverse event information.
Caution: Federal law restricts this device to sale by, or on the order of, a physician. Please reference the Directions for Use labeling for a complete list of contraindications, warnings, precautions, and adverse events.